Advances in Uropathology (2022–2025)
Over the past three years, there have been significant advances in the pathology of urologic organs – particularly the kidney, prostate, and bladder. Updated classification systems (notably the 2022 WHO 5th Edition), new molecular diagnostics, revised grading criteria, and translational research findings are reshaping diagnostic practice. Below, we summarize key developments for each organ system, focusing on clinically relevant changes in human pathology.
Kidney Pathology Updates (2022–2025)
Classification and Nomenclature: The WHO 2022 Classification of Urinary and Male Genital Tumors introduced major revisions for renal tumors. Important changes include a reorganization of renal cell neoplasms and recognition of new tumor entitiespathologyoutlines.compathologyoutlines.com:
Papillary RCC redefined: The traditional subdivision into type 1 vs. type 2 papillary RCC has been abolished. “Classic” papillary RCC now encompasses tumors that were formerly type 1, whereas many tumors previously labeled “type 2” are reclassified as distinct entities (e.g. fumarate hydratase–deficient RCC, acquired cystic disease–associated RCC, TFE3-rearranged RCC, etc.)pathologyoutlines.com. A provisional entity, papillary renal neoplasm with reverse polarity, has also been described for a rare papillary tumor variantpathologyoutlines.com. Papillary adenoma continues to be recognized as a separate, benign lesionpathologyoutlines.com.
Oncocytic tumor category: A new category “other oncocytic tumors” captures renal oncocytic neoplasms that fall between classic oncocytoma and chromophobe RCCpathologyoutlines.com. This includes emerging low-grade oncocytic tumor (LOT) and eosinophilic vacuolated tumor (EVT), as well as hybrid oncocytic tumors (often seen in Birt-Hogg-Dubé syndrome)pathologyoutlines.com. Chromophobe RCC itself is unchanged, but WHO now acknowledges unusual morphologies and “borderline” oncocytic tumors, reflecting growing recognition of gray-zone entities.
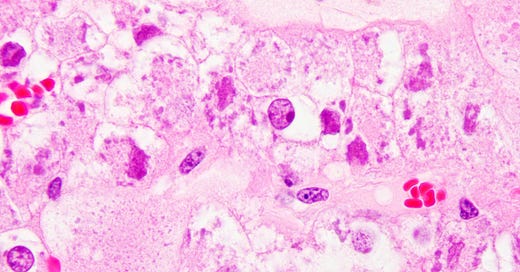
New tumor entities: Three novel RCC subtypes are formally recognized: eosinophilic solid and cystic RCC (ESC RCC), ELOC (TCEB1)-mutated RCC, and ALK-rearranged RCCpathologyoutlines.com. Each has distinct molecular drivers. ESC RCC is a recently described indolent tumor with solid and cystic architecture, voluminous eosinophilic cytoplasm and often mutations in TSC1/TSC2 (tuberous sclerosis genes)pathologyoutlines.com. ELOC-mutated RCC (also known as TCEB1-mutant RCC) is a clear-cell neoplasm characterized by inactivation of the ELOC gene (part of the VHL complex) instead of VHL itself, typically showing clear cells with fibromuscular bands and generally low metastatic potentialmodernpathology.org. ALK-rearranged RCC is defined by translocations involving ALK (e.g. VCL–ALK fusion), often occurring in younger patients; it can be identified by ALK immunohistochemistry and FISH, and is of interest because of potential response to ALK inhibitor therapypathologyoutlines.com.
Histology of Eosinophilic Solid and Cystic RCC (ESC RCC) – a newly recognized renal tumor subtype. Tumor cells have abundant eosinophilic cytoplasm with coarse basophilic cytoplasmic stippling/inclusions (H&E stain). This entity frequently harbors TSC1 or TSC2 mutationspathologyoutlines.com and shows solid and cystic growth patterns.
Molecularly-defined RCC: The classification now explicitly separates certain RCCs by molecular alterations. For example, the MiT family of translocation carcinomas has been split: TFE3-rearranged RCC and TFEB-altered RCC are listed as distinct entities (rather than lumped under a single MiT category)pathologyoutlines.compathologyoutlines.com. This change underscores the need for molecular testing (FISH or sequencing) to confirm the specific gene fusion involved. RCCs associated with hereditary syndromes (like FH-deficient RCC in HLRCC syndrome, SDH-deficient RCC, etc.) continue to be recognized, and the WHO now highlights “essential and desirable” diagnostic criteria, including molecular tests, for these tumorspubmed.ncbi.nlm.nih.gov.
Grading and Criteria: Grading of renal cell carcinomas has been refined. The WHO/ISUP nuclear grade is now assigned based on the single worst focus (highest-grade nucleus observed in one high-power field), rather than the predominant grade throughout the tumorpathologyoutlines.com. This “highest-grade focus” rule aligns with prior ISUP guidancepathologyoutlines.com. Notably, WHO emphasizes that grade is not equally relevant for all RCC subtypespathologyoutlines.com. For example, chromophobe RCC is no longer graded by the 1–4 nuclear grade system (since its prognosis is tied more to stage than nuclear features), and collecting duct carcinoma and renal medullary carcinoma are considered high-grade by definition. In contrast, clear cell and papillary RCC should be graded (as Grade 1–4) based on nucleolar prominence and pleomorphism, which correlates with outcomepathologyoutlines.com. These grading updates aim to standardize reporting and prognostication across institutionspathologyoutlines.com.
Immunohistochemistry and Molecular Diagnostics: Pathologists have an expanded toolkit of markers to classify renal tumors. The new entities often require ancillary testing for confirmation:
Translocation RCCs: TFE3 and TFEB translocation carcinomas can be screened by immunohistochemistry (TFE3 or TFEB protein overexpression) and confirmed by break-apart FISH or sequencing of gene fusionsnews-medical.net. Similarly, ALK-rearranged RCC is identified by ALK protein IHC (diffuse positive) and ALK gene break-apart probespathologyoutlines.com.
FH-deficient RCC: This hereditary-associated tumor (fumarate hydratase–deficient RCC) is diagnosed by loss of FH enzyme expression and positive intracytoplasmic 2SC (succination) staining on IHC, prompting genetic testing for HLRCC syndrome. Recognizing this entity is clinically crucial, as patients require surveillance for syndrome manifestationspathologyoutlines.com.
SDH-deficient RCC: Another rare variant requiring IHC: loss of SDHB (succinate dehydrogenase B) staining indicates an SDH-deficient tumor, associated with germline SDH mutations. This too has implications for genetic counseling.
ESC RCC: The ESC subtype can be suggested by morphology (eosinophilic cells with coarse basophilic inclusions) and confirmed by its unique immunoprofile (typically CK20-positive/CK7-negative, cathepsin-K positive in many cases)pathologyoutlines.compathologyoutlines.com. Molecularly, ESC RCC often shows TSC1 or TSC2 mutations, even in patients without tuberous sclerosispathologyoutlines.com. This connects ESC RCC to the mTOR pathway, and ongoing studies are examining if these tumors respond to mTOR inhibitor therapy.
ELOC-mutated RCC: These tumors resemble clear cell RCC but lack VHL mutations. If a clear-cell carcinoma shows unusual morphology (e.g., intersecting fibromuscular bands) and if genomic sequencing reveals TCEB1/ELOC mutation with intact VHL, it falls under this new categorymodernpathology.org. While not all centers will test for ELOC routinely, awareness of this entity helps avoid misclassifying such cases as conventional clear cell RCC.
Prognostic and Predictive Biomarkers: Beyond classification, research has advanced our understanding of RCC biomarkers:
Chromosome 3p mutations: Clear cell RCC can be stratified by mutations in chromatin-modifier genes on 3p. Notably, BAP1 mutations are associated with high grade, aggressive behavior, while PBRM1 mutations (often mutually exclusive with BAP1) tend to occur in lower-grade tumorspmc.ncbi.nlm.nih.govmdpi.com. Studies confirm that BAP1 loss correlates with worse outcomes and higher Fuhrman/ISUP grade, whereas PBRM1-mutant tumors are often less aggressivepmc.ncbi.nlm.nih.govmdpi.com. Although testing these genes is not yet routine in pathology reports, they are being used in research and may inform prognosis or eligibility for trials (for example, PBRM1 status has been explored as a predictor of immunotherapy responseascopubs.org).
Gene expression signatures: Molecular prognostic assays are emerging. A 16-gene expression score and the ClearCode34 assay have been validated to predict recurrence risk in localized clear cell RCCmdpi.commdpi.com. These assays stratify tumors into “good” vs. “poor” risk groups beyond traditional staging. While not standard of care yet, they exemplify the move toward precision prognostication.
Therapeutic markers: The expansion of systemic therapies for RCC (especially immunotherapies and targeted agents) has some impact on pathology. For instance, checkpoint inhibitor immunotherapy is now frontline for advanced RCC; unlike some other cancers, RCC’s response to immunotherapy does not require PD-L1 immunohistochemistry by current guidelines (PD-L1 is not a definitive predictive marker in RCC, as both PD-L1–positive and –negative RCC can respond). However, emerging research suggests tumor immune profiling and HLA genotype might influence immunotherapy outcomes. On the targeted therapy front, MET inhibitors are being used in MET-driven papillary RCC, so identifying papillary tumors with MET alterations (through sequencing or immunohistochemistry for MET) can be clinically relevant. Similarly, recognition of VHL disease-associated RCC (usually clear cell) is important, as such patients might be eligible for the recently approved HIF2α inhibitor therapy (belzutifan)news-medical.net.
Liquid biopsy: A forward-looking area is the use of circulating tumor DNA (ctDNA) in RCC. Historically, RCC is not the easiest cancer for liquid biopsy due to modest ctDNA shedding, but recent studies show promise. Detectable ctDNA in plasma correlates with tumor burden and may signal recurrence earlier than imagingmdpi.commdpi.com. Trials (e.g. NCT05287561) are ongoing to see if post-nephrectomy ctDNA can guide adjuvant therapy decisionsmdpi.commdpi.com. Though still investigational, in the next few years pathologists might work with oncologists to integrate ctDNA results for RCC monitoring.
Prostate Pathology Updates (2022–2025)
Classification and Terminology: The 5th edition WHO 2022 prostate tumor classification introduced evolutionary (but not revolutionary) changes. Many tweaks standardize terminology and incorporate new knowledge without dramatically altering diagnostic criteriaold.pathologica.it. Key updates include:
“Variants” vs. “Subtypes”: The terminology has been refined: distinct morphologic forms of acinar adenocarcinoma are now referred to as subtypes rather than “variants,” and characteristic patterns within a tumor are termed histologic patternsold.pathologica.it. For example, ductal adenocarcinoma is considered a subtype of prostatic adenocarcinoma (rather than a separate “variant”), as is mucinous (colloid) adenocarcinoma. This nomenclature change emphasizes that most of these are all prostate adenocarcinomas albeit with special growth patterns.
PIN-like adenocarcinoma: What was historically called “low-grade adenocarcinoma resembling high-grade PIN” has now been reclassified as a subtype of acinar adenocarcinomaold.pathologica.it. PIN-like carcinoma is characterized by large, rounded glands with papillary or tufted arrangements of cells that have minimal atypia (mimicking prostatic intraepithelial neoplasia). It behaves relatively indolently. The WHO acknowledges it as a true carcinoma subtype (Gleason pattern 3) rather than an in-situ lesion, since it lacks basal cells and can infiltrate locallyjpatholtm.org. Notably, recent studies found PIN-like carcinoma frequently harbors activating mutations in the RAS/MAPK pathway (RAF/RAS genes), a molecular clue that distinguishes it from benign PIN lesionsjpatholtm.org.
Histology of PIN-like Prostatic Adenocarcinoma, a newly defined subtype of acinar carcinoma. (A) H&E section shows large discrete glands with papillary infolding, resembling high-grade PIN but lacking significant cytologic atypia. (B) Immunohistochemistry for high-molecular-weight cytokeratin (34βE12) highlights an absence of basal cells in these glands, confirming invasive carcinoma. PIN-like carcinoma generally has a favorable prognosis and is often Gleason pattern 3jpatholtm.org.
Neuroendocrine Tumors – new category: The WHO now recognizes “treatment-related neuroendocrine prostatic carcinoma (t-NEPC)” as a distinct tumor typeold.pathologica.it. This refers to aggressive neuroendocrine carcinomas (usually small cell or mixed small cell) that arise after hormonal therapy for typical prostate adenocarcinoma. Such tumors often present in the castration-resistant stage, showing small cell morphology or other NE features with loss of androgen receptor expression. Previously, small cell carcinoma of the prostate was described without context; the new designation t-NEPC underlines the common clinical scenario of therapy-related emergenceold.pathologica.itold.pathologica.it. It has important therapeutic implications: t-NEPC is managed with platinum-based chemotherapy rather than standard hormonal therapy. Notably, in the WHO classification scheme, general neuroendocrine neoplasms (small cell carcinoma, well-differentiated NET, etc.) are consolidated in a separate chapter for all organs, except t-NEPC remains in the prostate chapter because of its unique clinical contextold.pathologica.itold.pathologica.it.
Other nomenclature changes: Basal cell carcinoma of the prostate (a very rare tumor) has been renamed to adenoid cystic carcinoma of the prostatejpatholtm.org. The tumor is histologically analogous to salivary adenoid cystic carcinoma, and the new name avoids confusion with cutaneous basal cell carcinoma. Additionally, WHO 2022 incorporates stromal tumors of the prostate (e.g. stromal tumor of uncertain malignant potential) but those saw no major change. Rare entities like prostatic squamous cell carcinoma and adenosquamous carcinoma remain recognized, and mesenchymal tumors common to the GU tract are grouped in separate sections.
Grading and Diagnostic Criteria: The Gleason grading system (now typically expressed as Grade Groups 1–5) remains the cornerstone for prostate cancer. However, several grading issues have seen debate and clarification in recent years:
Intraductal carcinoma of the prostate (IDC-P): IDC-P is a proliferation of malignant cells filling prostatic ducts/acini with preservation of some basal cells. It is strongly associated with high-grade invasive cancer. There has been controversy on whether to assign Gleason grades to IDC-P when it co-occurs with invasive cancer. Leading expert groups diverge: The International Society of Urological Pathology (ISUP) suggests including IDC-P in the overall Gleason score (i.e. consider the patterns of IDC-P as part of grading) and always reporting its presence due to adverse prognosisjpatholtm.orgjpatholtm.org. In contrast, the Genitourinary Pathology Society (GUPS) recommends not to incorporate IDC-P into the Gleason grade, arguing it should be noted separately (since it’s an in-situ process)pmc.ncbi.nlm.nih.gov. WHO 2022 acknowledges the lack of consensus – it directs pathologists to report IDC-P’s presence, but leaves the grading approach open, noting the conflicting guidelinesjpatholtm.orgjpatholtm.org. In practice, most agree IDC-P should be prominently mentioned in reports because it portends a worse outcome (and may influence therapy decisions, such as prompting definitive treatment even if invasive cancer volume is low)pmc.ncbi.nlm.nih.gov.
Cribriform carcinoma: There is now broad agreement that cribriform Gleason pattern 4 is an especially ominous pattern. Multiple studies (2017–2022) showed cribriform morphology in prostate cancer correlates with higher risk of progression and metastasis, independent of Gleason score. As a result, pathologists are recommended to comment on cribriform pattern presence. WHO 2022 stops short of changing grading definitions but supports the notion that cribriform architecture is prognostically adverseold.pathologica.it. Many clinicians will avoid active surveillance if cribriform pattern is reported in even Grade Group 2 tumors, for example. A related change: the entity formerly termed “cribriform PIN” (basically a proliferation with larger cribriform spaces than typical HGPIN) is now regarded as part of a continuum between HGPIN and IDC-P, often termed atypical intraductal proliferation (AIP) rather than a distinct diagnosisjpatholtm.org. This acknowledges that some lesions are equivocal between HGPIN and IDC-P; if truly worrisome cribriform proliferation is seen, one should look hard for invasive cancer.
Reporting of tertiary grades: In Grade Group 1–5, prostate cancers are graded by the two most prevalent patterns. However, a tertiary (third) pattern can be present in prostatectomy specimens. Specifically, a minor component of Gleason pattern 5 in an otherwise lower-grade tumor is of clinical significance. Recent ISUP consensus (2019) recommended always reporting any tertiary pattern 5 in prostatectomy, and some experts even advocate upgrading certain cases to reflect that minor component. WHO 2022 acknowledges this issue but did not formally change the grading algorithmold.pathologica.it. The College of American Pathologists (CAP) protocol currently advises: for prostatectomy, if <5% of the tumor is Gleason 5, one should still report the primary+secondary as the Gleason score but mention the tertiary 5; if ≥5% is pattern 5, then that pattern should be included in the Gleason score calculation as the secondary pattern. On needle biopsy, any amount of pattern 5 should be included in the Gleason score (due to undersampling). These practices are aimed at reflecting tumor biology more accurately in the report.
Molecular and Clinical Advances: Prostate cancer diagnostics are increasingly augmented by molecular tests, especially in the context of prognostication and therapy selection:
Genomic risk classifiers: Several commercial gene-expression tests have gained traction to guide management of localized prostate cancer. For example, the Decipher genomic classifier (22-gene signature) and others like Oncotype DX Prostate and Prolaris are now backed by validation studies that correlate their score with risk of metastasis. Notably, data from 2020–2023 have shown the Decipher score predicts which post-prostatectomy patients benefit from adjuvant therapycxbladder.com. As a result, genomic classifiers have been incorporated into NCCN guidelines as optional tools in deciding adjuvant radiation or in selecting candidates for active surveillance. While these tests are performed on prostatectomy or biopsy tissue (often sent out to specialized labs), pathologists play a role by providing appropriate tissue blocks and interpreting results in tumor board discussions.
Homologous recombination repair (HRR) gene testing: A major development in advanced prostate cancer is the recognition of DNA repair gene mutations (like BRCA1, BRCA2, ATM, CHEK2, PALB2, etc) as both prognostic and predictive markers. Approximately 20% of metastatic castration-resistant prostate cancers (mCRPC) harbor an alteration in one or more HRR genes. Clinical trials (PROfound and others) demonstrated that PARP inhibitors (e.g. olaparib, rucaparib) significantly improve outcomes in mCRPC patients with BRCA1/2 or certain other gene mutations. Consequently, professional guidelines now recommend molecular testing for HRR gene mutations in advanced prostate cancerjpatholtm.orgjpatholtm.org. This can be done via tumor tissue sequencing or liquid biopsy. From a pathology standpoint, this means that for patients with high-grade or metastatic prostate cancer, the pathology report or the oncologist may request next-generation sequencing (NGS) on the tumor to detect mutations in BRCA1/2 and related genes. If a deleterious mutation is found, the patient may be a candidate for PARP inhibitor therapy. Additionally, the presence of an MMR (mismatch repair) deficiency in prostate cancer, though rare (~3–5% of advanced cases), predicts response to PD-1 checkpoint inhibitors (Pembrolizumab). Therefore, many centers now perform MSI or MMR IHC testing on metastatic prostate tumors or in patients with a strong family history. The WHO notes these therapeutic correlations: prostate cancers with homologous recombination defects respond to PARP inhibitors, and those with MMR defects may respond to immunotherapyjpatholtm.org.
Androgen receptor splice variant 7 (AR-V7): An evolving predictive biomarker in mCRPC is AR-V7, an androgen receptor variant detected in circulating tumor cells. AR-V7 positivity in the blood indicates likely resistance to AR-targeted therapies (like enzalutamide and abiraterone). By 2022, a CLIA-certified blood test for AR-V7 (Epic Sciences) became available and was incorporated into some guidelines: if an mCRPC patient is AR-V7 positive, clinicians are advised to use chemotherapy or other options rather than another AR signaling inhibitorepicsciences.combmccancer.biomedcentral.com. While this is more in the realm of medical oncology than surgical pathology, it reflects the expanding use of liquid biopsy biomarkers. Pathologists should be aware of AR-V7 testing as part of the multidisciplinary care in advanced cases, although they typically do not perform this test themselves.
PSMA and theranostics: A breakthrough in prostate cancer imaging/treatment has been the rise of PSMA PET scans and PSMA-targeted radioligand therapy (e.g. ^177Lu-PSMA-617). Nearly all prostate acinar adenocarcinomas overexpress PSMA (prostate-specific membrane antigen) on the cell surface. Pathologists have long used PSMA immunohistochemistry as a sensitive marker to identify prostatic origin of metastases. Now, that same target is used to deliver imaging agents and radionuclide therapy. The approval of PSMA PET in 2021 and radioligand therapy in 2022 doesn’t change how we diagnose prostate cancer, but it reinforces the importance of recognizing even low-grade prostate cancer foci (since molecular imaging can detect very small lesions) and of reporting any neuroendocrine differentiation (which usually has low PSMA expression and might not respond to PSMA-targeted approaches). Additionally, pathologists may occasionally get biopsies of unusual metastases (e.g. a lung nodule) where they confirm prostate origin by PSMA IHC, helping direct patients to PSMA-based treatments.
Artificial intelligence (AI) in prostate pathology: An emerging practical advance is the use of AI algorithms for prostate biopsy interpretation. In 2022, several AI-based pathology tools received regulatory approval or came close. For instance, FDA-authorized AI platforms can assist in detecting prostate cancer on digitized slides and even assign Gleason grades. Studies presented at USCAP 2023 demonstrated that AI can catch minute cancer foci that might be missed and help standardize grading, though these tools are meant to complement, not replace, pathologist review. Some high-volume labs have started implementing AI-assisted screening of prostate cores as a quality step. This trend is likely to grow, reducing diagnostic turnaround times and potentially increasing consistency in Gleason grading.
Notable Research/Conference Highlights: Recent major conferences have spotlighted topics like the impact of tumor genetics on treatment. At ASCO GU 2023 and EAU 2023, for example, there was emphasis on sequencing metastatic prostate tumors for actionable mutations (HRR and MSI as mentioned). The ISUP 2022 meeting held a consensus session on grading cribriform cancer and tertiary patterns, feeding into the ongoing grading debatespmc.ncbi.nlm.nih.gov. Meanwhile, long-term data from active surveillance cohorts (presented at AUA and EAU meetings) continue to refine selection criteria – reinforcing that Grade Group 1 and some very limited Grade Group 2 cancers can be safely observed, but any cribriform pattern or IDC-P should generally prompt treatment. These insights, while clinical, rely on pathologists accurately identifying those adverse features on biopsies.
In summary, prostate pathology has seen its WHO classification fine-tuned and its role expanded to include molecular testing coordination. The diagnostic criteria have been clarified in areas like PIN-like carcinoma and neuroendocrine carcinoma. Molecular pathology is now central to advanced prostate cancer care, bridging the gap between biopsy diagnosis and personalized therapy selection.
Bladder (Urothelial) Pathology Updates (2022–2025)
Classification and Grading: Urothelial (bladder) tumors have a revised framework in the WHO 5th Edition (2022), incorporating both histologic refinements and emerging molecular classification. Some of the key updates in classification and terminology are:
Non-invasive papillary lesions: Two previously described precursor lesions – papillary urothelial hyperplasia and urothelial proliferation of uncertain malignant potential (UPUMP) – are no longer separate entities. WHO now considers these as part of the spectrum of early low-grade papillary urothelial carcinoma rather than benign or “undetermined” lesionsjpatholtm.orgjpatholtm.org. In practice, this means a papillary lesion with minimal atypia and architecture not overtly carcinoma should be categorized as low-grade papillary carcinoma (with a comment if needed) rather than a vague “proliferation” diagnosis.
Papillary urothelial neoplasm of low malignant potential (PUNLMP): The 5th edition retains PUNLMP as a valid diagnostic categorypmc.ncbi.nlm.nih.gov, despite its declining usage. PUNLMP refers to papillary lesions that have thicker urothelium than papillomas but lack significant cytologic atypia. They have a very low risk of progression (lower than low-grade carcinoma)pmc.ncbi.nlm.nih.gov. The WHO editors kept PUNLMP to avoid labeling such indolent lesions as “carcinoma” in pathology reports, which can spare patients anxietypmc.ncbi.nlm.nih.gov. However, practically, PUNLMP diagnoses are rare (studies show it now comprises <2% of papillary tumor diagnoses)pmc.ncbi.nlm.nih.gov and many experts expect it may eventually be merged into low-grade carcinomapmc.ncbi.nlm.nih.gov. For now, pathologists should use the PUNLMP diagnosis only when the patient has no prior history of urothelial carcinoma and the lesion meets strict morphologic criteria (exophytic papillae with virtually no atypia)pmc.ncbi.nlm.nih.gov.
New grading criteria for mixed-grade papillary tumors: Recognizing that some papillary urothelial carcinomas show predominantly low-grade features with focal high-grade areas, WHO 2022 provides clearer guidance. A papillary tumor with ≥5% high-grade component should be classified as “high-grade” overalljpatholtm.orgjpatholtm.org. If the high-grade component is truly minimal (<5% of the lesion), the tumor can be diagnosed as “low-grade papillary urothelial carcinoma with <5% high-grade component”jpatholtm.org. This approach addresses intra-tumor heterogeneity and ensures that even a small high-grade focus is noted, which is clinically relevant (small high-grade areas can impact surveillance intervals and intravesical therapy decisions). Major urologic guidelines (EAU, AUA) still accept either the 1973 WHO grading (G1/G2/G3) or 2004/2016 WHO grading (low vs high), but this refined reporting helps convey nuance within the 2-tier systemuroweb.org.
Flat lesions (CIS and dysplasia): Urothelial carcinoma in situ (CIS) remains defined as before (high-grade flat neoplasm). One minor change: WHO no longer gives “urothelial dysplasia” its own separate section, though the term dysplasia can still be used descriptively for a flat atypical urothelium falling short of CISjpatholtm.org. In practice, the Paris System for urine cytology and many pathologists already use “atypical urothelial cells” for indeterminate cases and reserve “dysplasia” rarely for surgical samples with atypia not qualifying as CIS. The diagnostic threshold for CIS remains the same (significant nuclear atypia in the flat urothelium, often full-thickness). Notably, any confirmed CIS is high grade by definition.
Invasive urothelial carcinoma variants: The new WHO continues to list various histologic variants (subtypes) of invasive urothelial carcinoma (e.g. micropapillary, plasmacytoid, sarcomatoid, nested variant, etc.), as these have clinical importance. One change is more of emphasis: variants are now called “histologic patterns” or “subtypes” rather than separate entities, to encourage reporting of mixed forms. For example, a tumor can be diagnosed as “Invasive urothelial carcinoma with micropapillary pattern” when such component is present, as this pattern signifies a higher stage at presentation and may influence therapy (micropapillary bladder cancer often prompts early cystectomy and consideration of systemic therapy). The WHO notes that any “unique morphology with prognostic significance” should be termed a tumor subtype, whereas purely descriptive differences are just patternsjpatholtm.org.
Non-urothelial tumors: While not the focus of this question, it’s worth noting WHO 2022 includes updates on less common bladder tumors (e.g. a new category for large cell neuroendocrine carcinoma of bladder, etc.). But the vast majority of bladder malignancies are urothelial, so the primary updates revolve around urothelial lesions.
Molecular Advances and Biomarkers: Perhaps the most notable recent progress in bladder cancer is the integration of molecular data for classification and management:
Consensus molecular subtypes of muscle-invasive bladder cancer: Multiple genomic studies over the last decade converged on the concept of distinct molecular subtypes of bladder cancer, analogous to breast cancer subtypes. In 2020, an international consortium published a consensus molecular classification of muscle-invasive bladder cancer (MIBC), identifying six molecular subgroups based on mRNA expression profilespmc.ncbi.nlm.nih.govjpatholtm.org. These subtypes are: Luminal-Papillary (~24%), Luminal-Nonspecified (~8%), Luminal-Unstable (~15%), Stroma-rich (~15%), Basal/Squamous (~35%), and Neuroendocrine-like (~3%)jpatholtm.org. Each subtype has unique biology and outcome: for instance, luminal-papillary tumors often have FGFR3 mutations and a better prognosis, basal-squamous tumors have aggressive behavior but may respond better to immunotherapy, and neuroendocrine-like tumors include small cell cancerspmc.ncbi.nlm.nih.govjpatholtm.org. The WHO 2022 text discusses these molecular subtypes as an important research development, although it stops short of requiring subtype assignment on pathology reports. The challenge is that determining these subtypes requires gene expression profiling (mRNA analysis), which is not yet routine in clinical practice due to cost and complexitypmc.ncbi.nlm.nih.gov. However, proxy methods are being explored: a simplified panel of immunohistochemical stains (e.g. GATA3, CK20, uroplakin II for luminal differentiation; CK5/6 and CK14 for basal differentiation) can classify many tumors into luminal vs basal categoriespmc.ncbi.nlm.nih.gov. Such IHC-based subtyping has about 70–80% concordance with molecular subtypes and could be an accessible tool for pathologistspmc.ncbi.nlm.nih.gov. Overall, the recognition of molecular subtypes is influencing clinical trials and therapy (for example, luminal tumors might benefit from FGFR inhibitors if FGFR3-mutated, while basal tumors may benefit from added chemotherapy). Pathologists should be aware of these categories and perhaps note in reports if a tumor shows features of a particular subtype (some centers now add comments like “Tumor shows basal/squamous features by immunoprofile,” etc., especially in academic settings).
Genomic markers for therapy: Several actionable genetic alterations have been identified in urothelial carcinoma, and testing for them is increasingly important:
FGFR3 mutations/fusions: Approximately 15–20% of advanced urothelial carcinomas (especially upper tract and luminal papillary tumors) have activating FGFR3 gene alterations. In 2019, the FDA approved erdafitinib (an FGFR inhibitor) for metastatic urothelial carcinoma with FGFR3 or FGFR2 alterations. Therefore, whenever a high-grade urothelial carcinoma is diagnosed in a metastatic or recurrent setting, molecular testing for FGFR alterations should be considered. Many institutions now reflexively test any muscle-invasive bladder tumor for FGFR3 mutations (via targeted NGS panels). The presence of an FGFR3 mutation can direct second-line therapy to erdafitinib. The WHO highlights this by noting FGFR3-altered tumors may be eligible for FGFR inhibitor treatmentjpatholtm.orgjpatholtm.org.
DNA damage repair genes: Mutations in genes like ERCC2, ERCC1, and others involved in nucleotide excision repair have been correlated with increased sensitivity of urothelial carcinoma to cisplatin-based chemotherapy. For example, ERCC2 mutations are enriched in responders to platinum chemo (because defective DNA repair makes tumor cells more susceptible to DNA-damaging agents). The WHO mentions that alterations in ERCC2 or other DNA repair genes may predict which tumors are likely to benefit from cisplatin chemotherapyjpatholtm.org. While routine testing of ERCC2 is not yet standard, this finding is being investigated to personalize neoadjuvant chemo decisions. Some commercial tests (like molecular subtyping assays) incidentally report if a tumor has a DNA repair gene mutation.
PD-L1 and immune biomarkers: Checkpoint immunotherapy (anti PD-1/PD-L1) is now a pillar of advanced bladder cancer treatment. PD-L1 immunohistochemistry is used in certain clinical scenarios – for instance, in metastatic patients who are cisplatin-ineligible, a PD-L1 test (using an FDA-approved antibody clone and scoring system) is needed to determine if they can get upfront immunotherapy. WHO notes that PD-L1 expression in tumor and immune cells is one predictor of response to checkpoint inhibitorsjpatholtm.org. Other emerging predictors include tumor mutation burden (TMB) and microsatellite instability (MSI) statusjpatholtm.org. High TMB in bladder cancer has been associated with better immunotherapy outcomes (bladder cancers tend to be high TMB on average due to their mutagenic exposures like tobacco). MSI-H is rare in bladder cancer (<2%) but if present, also portends a strong response to PD-1 blockade. As a result, some panels now test bladder cancers for MSI or mismatch repair proteins, especially if there’s a clinical suspicion of Lynch syndrome or an exceptionally high TMB noted on sequencing. From a pathologist’s perspective, the main test performed is PD-L1 IHC on either the resection or a metastatic biopsy, using assays like 22C3 or SP142 depending on the therapy. It’s important to report PD-L1 results with the proper scoring (e.g. Combined Positive Score or Tumor-Infiltrating Immune Cell score) as required. The field is moving toward composite biomarkers; for now PD-L1 IHC is entrenched in practice for certain indications.
TERT promoter mutations: One of the most ubiquitous mutations in bladder cancer (especially non-invasive papillary tumors) is in the TERT promoter (over 70% of bladder tumors have it). While this doesn’t change classification, it has practical diagnostic utility. The WHO mentions that finding a TERT promoter mutation in urothelial cells can help confirm that atypical cells are truly neoplastic (as opposed to reactive)jpatholtm.orgjpatholtm.org. For example, if one has a borderline lesion or equivocal urine cytology, detection of TERT promoter mutation strongly favors an underlying urothelial carcinoma. Several urine-based assays capitalize on this: e.g. the UroSEEK urine test (developed at Johns Hopkins) evaluates mutations in TERT and FGFR3 plus others to detect bladder cancer in urine with high sensitivity. In tissue, while we don’t test TERT routinely, a difficult case of atypical hyperplasia vs. low-grade carcinoma could theoretically be resolved by finding a TERT mutation. The presence of clonal mutations (like TERT) in an area of urothelium also supports that field as neoplastic.
Advances in Urine-Based Diagnostics: Because bladder cancer is often multifocal and recurrent, non-invasive urine tests have long been a focus of research. In the last few years, urine molecular tests have made meaningful progress:
Traditional urine cytology (while very specific for high-grade disease) has low sensitivity for low-grade tumors. FDA-approved urine assays like NMP22, BTA-Stat, and UroVysion FISH have been available, but with variable performancefrontiersin.orgfrontiersin.org. Recently, newer tests that use genomic markers have shown improved accuracy. Notably, the Cxbladder test (a multi-gene mRNA expression assay on urine) underwent a prospective trial (the STRATA study) which demonstrated that its “Triage” version could safely rule out cancer in low-risk hematuria patients, reducing the need for unnecessary cystoscopiescxbladder.comcxbladder.com. Based on this evidence, in 2025 the American Urological Association (AUA) updated its guidelines to incorporate urine biomarkers in the evaluation of hematuria. The guideline now states that in intermediate-risk hematuria patients (e.g. microhematuria with risk factors), a clinician may offer a validated urine test (like Cxbladder) in lieu of immediate cystoscopy, if the patient understands the trade-offscxbladder.comcxbladder.com. Cxbladder Triage is specifically cited as the only test with Level 1 (Grade A) evidence from an RCT supporting its usecxbladder.com. This is a significant milestone – it’s one of the first times a urine biomarker has been written into a urology guideline based on high-level evidence. Practically, this means pathologists/clinical labs are increasingly involved in running such molecular urine tests. Other promising urine tests include Xpert Bladder Cancer Monitor (a PCR-based assay detecting gene expressions) and AssureMDx (mutation/methylation assay). While these aren’t yet guideline-endorsed, they are used by some clinicians to monitor for tumor recurrence, potentially reducing frequency of cystoscopies if negative.
The Paris System for Urine Cytology was updated in a second edition (2022), refining cytologic criteria to reduce “atypical” diagnoses and better correlate with molecular findings. For instance, the category of “atypical urothelial cells” is now more strictly defined, and any specimen with urothelial cells showing abnormal chromatin should prompt consideration of UroVysion FISH or a molecular test to detect occult high-grade disease. The integration of cytology with reflex molecular testing (e.g. if cytology is atypical, do FISH or a DNA test) is a workflow being adopted in some centers.
Translational Research and Therapeutic Impact: Several recent clinical findings are important for pathologists to note:
Adjuvant therapy in high-risk bladder cancer: Results of trials like IMvigor010 (adjuvant atezolizumab) and CheckMate 274 (adjuvant nivolumab) in 2021–2022 have led to checkpoint inhibitors being used after cystectomy in high-risk patients (especially if they didn’t get neoadjuvant chemo or have residual disease). Pathologists should ensure thorough assessment of cystectomy specimens for adverse features (lymphovascular invasion, extensive CIS, etc.), as these are criteria for “high-risk” that may prompt adjuvant therapy. Also, emerging data suggest that molecular residual disease (ctDNA) post-surgery is a strong predictor of recurrencemdpi.com. Ongoing trials (e.g. the MODERN trial) are testing adjuvant immunotherapy guided by ctDNA statuseuropeanurology.com. In future, a pathologist’s report might incorporate both anatomic pathology and a post-op blood-based ctDNA result to guide therapy.
Antibody-drug conjugates (ADCs): New therapies like enfortumab vedotin (which targets Nectin-4) and sacituzumab govitecan (targets Trop-2) have shown efficacy in advanced urothelial carcinoma (approved in 2019 and 2021 respectively for post-platinum, post-immunotherapy cases). These targets are highly expressed in most urothelial carcinomas. Pathologists do not currently test for Nectin-4 or Trop-2, since expression is usually ubiquitous enough. However, if resistance patterns emerge or patient selection is needed, IHC for these might become relevant. So far, no biomarkers are required for ADC use – virtually all urothelial carcinomas qualify.
Variant histology and treatment: Some bladder cancer variants prompt specific management. For instance, micropapillary urothelial carcinoma often warrants early cystectomy and consideration of HER2-targeted therapy in trials (as ~40% have ERBB2 amplification, similar to breast cancer). Plasmacytoid (signet-ring cell) variant is associated with CDH1 (E-cadherin) mutations; its identification is crucial as it often presents at advanced stage and may benefit from aggressive upfront therapy. These insights were highlighted in pathology conferences like USCAP and ISUP Controversies meetings, urging pathologists to always mention any variant component in the report. The WHO’s stance is that any fraction of variant histology should be reported, because even a 10% variant can dominate the clinical course (e.g. a mostly conventional tumor with 10% micropapillary component should still be flagged).
Sub-staging of T1 disease remains an unresolved issue. Some experts advocate reporting depth of lamina propria invasion (T1a vs T1b) based on muscularis mucosae level, given its prognostic importance, but reproducibility is a concern. GUPS published recommendations (2020) on handling T1 bladder tumors, suggesting measurement of invasion depth or quantification of invasive carcinoma volume. WHO 2022 did not mandate a specific T1 subclassification, but this topic has been prominent at urology and pathology forums. For now, many pathologists include a comment on extensive vs. focal lamina propria invasion in T1 tumors.
In summary, bladder cancer pathology has evolved with refined grading criteria and a nod to molecular classifications. Pathologists are increasingly engaged in multimodal diagnostics – from identifying subtle histologic changes to coordinating molecular tests on tissue and urine. The recent WHO update and conference consensus guidelines aim to improve diagnostic accuracy (reducing “undetermined” categories, clarifying borderline lesions) and to ensure that clinically significant findings (variant histology, high-grade foci, molecular markers) are reported. All these advances ultimately support more personalized management of bladder cancer, aligning pathology with therapeutic decision-makingjpatholtm.orgjpatholtm.org.
Sources:
WHO Classification of Urinary and Male Genital Tumors, 5th ed. (2022) – PathologyOutlines summary of updatespathologyoutlines.compathologyoutlines.com; WHO Blue Book chapters on Kidney, Prostate, Bladder.
Surintrspanont & Zhou. Pathologica. “Prostate Pathology: What is New in the 2022 WHO Classification…” (2023)old.pathologica.itold.pathologica.it.
Zynger et al. J Pathol Transl Med. “What’s New in Genitourinary Pathology 2023: WHO 5th Edition updates” (2024) – Bladder and Prostate sectionsjpatholtm.orgjpatholtm.org.
Recent literature on molecular and prognostic markers: e.g. Mubarak & Rashid. J Clin Transl Pathol. on WHO 2022 Kidney tumorsnews-medical.net; Mattila et al. Cancers (Basel) 2022 on RCC prognostic factorsmdpi.com; Lobo et al. Mod Pathol. 2020 on ELOC-mutated RCCmodernpathology.org; Biomarker reviews in Front Oncol 2024 for bladderfrontiersin.org.
Clinical guidelines and trials: AUA Microhematuria Guideline 2025 updatecxbladder.comcxbladder.com; NCCN/EAU Guidelines 2023 for prostate and bladder (genetic testing recommendations)jpatholtm.org; KEYNOTE-564, CheckMate 274 trials; Consensus molecular classification of bladder cancer (Kamoun et al., Eur Urol 2020)pmc.ncbi.nlm.nih.gov.
Conference highlights: USCAP 2022–2023 abstracts on prostate grading and AI; ASCO GU and AUA 2023 sessions on ctDNA in bladder cancereuropeanurology.com; ISUP and GUPS consensus statements on prostate grading and bladder pathologypmc.ncbi.nlm.nih.govsciencedirect.com.